Calcium
Calcium is the most abundant cation in the body,
Appearing in combination with phosphorus in the ratio 2:1.5
It is essential for
- the formation of bones and teeth,
- blood clotting,
- normal muscle and nerve activity,
- endocytosis and exocytosis,
- cellular motility,
- chromosome movement prior to cell division,
- glycogen metabolism,
- and synthesis and release of neurotransmitters.
Sources include
- milk and milk products,
- egg yolk,
- shellfish,
- and green leafy vegetables.
Most - 99% - is stored in the bones and teeth.
The remainder is stored in muscle, other soft tissues,
and blood plasma.
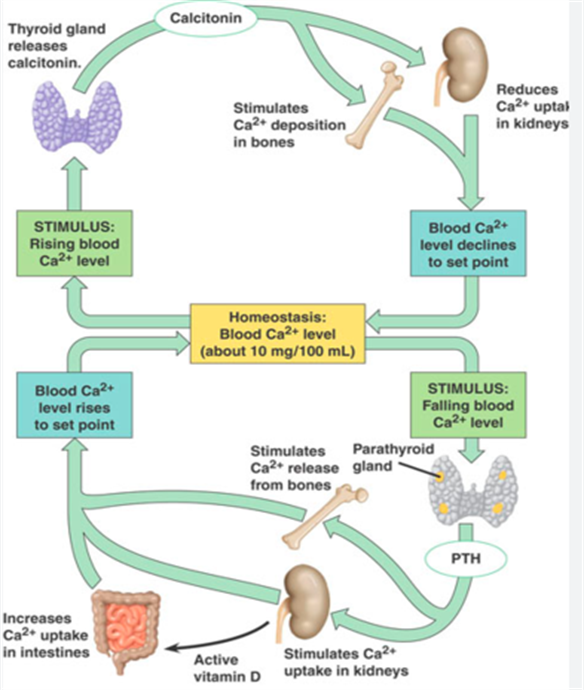
Regulation of plasma levels is by
- free exchange with body stores,
- and through the action of hormones such as
Calcitonin, Parathyroid hormone and Vitamin D.
Excess calcium is mostly excreted in faeces,
and in small amounts in urine.
Regulation
Given the range of roles for calcium within the body,
it is important to maintain its free extracellular concentration within narrow limits.
Assuming an adequate supply of dietary calcium, this is achieved through:
1. Efficient gastrointestinal absorption
2. Free exchange with bone stores:
the most important buffer for immediate changes of calcium concentration
3. Renal excretion of calcium
Hormonal influences on the preceding functions:
Vitamin D;
Promotes calcium and phosphate absorption within the gut
Promotes mineralization of bone taking up calcium and phosphate
Calcitonin aims to reduce serum calcium
Promotes mineralization of bone taking up calcium and phosphate
Parathyroid hormone aims to increase serum calcium
Promotes increased resorption of bone, causing release of
Reduces renal excretion of calcium
(Promotes kidney reabsorption of calcium, but not of phosphate)
Additionally, there is a small amount of calcium excretion within sweat
which become more significant in extremes of heat and low humidity.
Interaction calcium and phosphate
Calcium and phosphate ion physiology is closely related:
if the product of the concentration of each ion exceeds a value
that is termed their solubility product,
they precipitate out of solution.
This can result in new mineralization within bone or ectopic calcification.
Conversely, the equilibrium results in one ion being reabsorbed from bone
if the concentration of the other drops.
Therefore, a large drop in plasma phosphate concentration can cause hypercalcaemia.
Parathyroid hormone causes renal reabsorption of calcium but reduces phosphate reabsorption
1,25-dihydrocholecalciferol
promotes gastrointestinal absorption of both calcium and phosphate
However, unlike the free, ionized plasma concentration of calcium,
the concentration of ionized phosphate fluctuates considerably throughout the day.

